Soft tissue augmentation interventions are indicated to correct deformities of the oral mucosa in periodontal, peri-implant and edentulous sites. Autogenous soft tissue grafts are generally favored for such therapeutic purposes due to their superior biological properties and overall clinical performance compared to graft substitutes. Although the palatal region between the canine and first molar has been historically considered the primary source of intraoral autogenous soft tissue grafts, mainly due to accessibility and its dimensional configuration, there are other possible intraoral sources with distinct biological properties and several harvesting techniques that can be leveraged to facilitate the procedural execution and maximize treatment outcomes.
INTRODUCTION
A graft can be simply defined as a transplanted piece of living tissue used to correct a deformity. Although both terms are often used interchangeably, “grafts” are not the same as “graft substitutes”, which are exogenous materials of biologic or synthetic origin that do not contain native cells. Bone and soft tissue grafts and substitutes are a core component of contemporary periodontal and implant related plastic and reconstructive surgery (Chambrone & Avila-Ortiz, 2022). Autogenous soft tissue grafts and substitutes are applied in contemporary clinical practice for a wide variety of therapeutic purposes, the main indications being periodontal and peri-implant phenotype modification (i.e., keratinized tissue width, gingival/mucosal thickness and/or supracrestal tissue height augmentation) and treatment of gingival recession and peri-implant marginal mucosa defects .(Chambrone et al., 2019; Gamborena & Avila-Ortiz, 2021; Tavelli et al., 2020).
Although autogenous soft tissue grafts are associated with several disadvantages related to the need of harvesting the graft from a donor area (e.g., hemorrhage, increased surgical time, greater postoperative morbidity, lower patient acceptance, and, in some cases, limited tissue volume to perform the planned intervention), they are generally preferred for soft tissue augmentation interventions due to their superior biological properties (e.g., biocompatibility, revascularization potential, and supportive role as a scaffold for cellular migration and proliferation) and overall long-term clinical performance compared to graft substitutes (Chambrone et al., 2022; Chambrone & Tatakis, 2015).
The use of autogenous subepithelial connective tissue grafts (CTGs) for a wide variety of therapeutic purposes has been extensively tested and documented over the past several decades (Zucchelli et al., 2020). To achieve successful and predictable outcomes it is of paramount importance for the clinician to have a detailed knowledge of the biologic and structural characteristics of soft tissue grafts obtained from different intraoral donor areas, harvesting techniques available, as well as proper selection of the most appropriate surgical technique for a given clinical situation (e.g., tunnel approach for root coverage).
BIOLOGICAL CONSIDERATIONS
Mounting evidence regarding the structural biology of the periodontium and its surrounding structures has steered clinicians to personalized periodontal therapy, based on sound biological principles rather than purely personal preference and trends.
Although the palatal region between the maxillary canine and the first molar has been historically considered the primary source, CTGs may be harvested from different intraoral locations, such as the anterior palate, posterior palate, maxillary tuberosity and retromolar pad. The clinical outcomes of soft tissue augmentation procedures employing autogenous grafts may be largely impacted by the choice of the donor site. For instance, compared to CTGs obtained from the lateral palate, those harvested from the maxillary tuberosity typically demonstrate excellent dimensional stability and minimal post-operative shrinkage, with some even exhibiting a hyperplastic behavior over time (Pohl & Gluckman, 2024).
While the biological mechanisms behind these phenomena are yet to be fully elucidated, recent studies on the structural and molecular biology of CTGs harvested from different intraoral sources have revealed unique morphometric and cellular differences between them (Garcia-Caballero et al., 2023; Sanz-Martin et al., 2019; Stuhr et al., 2023), which could partially explain some variations in the phenotypic response after performing soft tissue augmentation interventions.
Although it has been shown that the structural composition of donor sites varies between and within individuals, the lamina propria of grafts from the tuberosity is usually denser and thicker compared to that of the anterior and posterior palate (Dellavia et al., 2014). As shown in Figure 1, the submucosal layer is less pronounced or even absent in the tuberosity and retromolar pad region, translating to a more organized, and less vascular and glandular structure (Stuhr et al., 2023). On the contrary, the deeper regions of the palatal mucosa present a greater proportion of submucosa, mainly in the canine and premolar area. When selecting a donor site, clinicians should ponder these properties, depending on the existing periodontal phenotype, the technique to be applied and specific needs of the patient.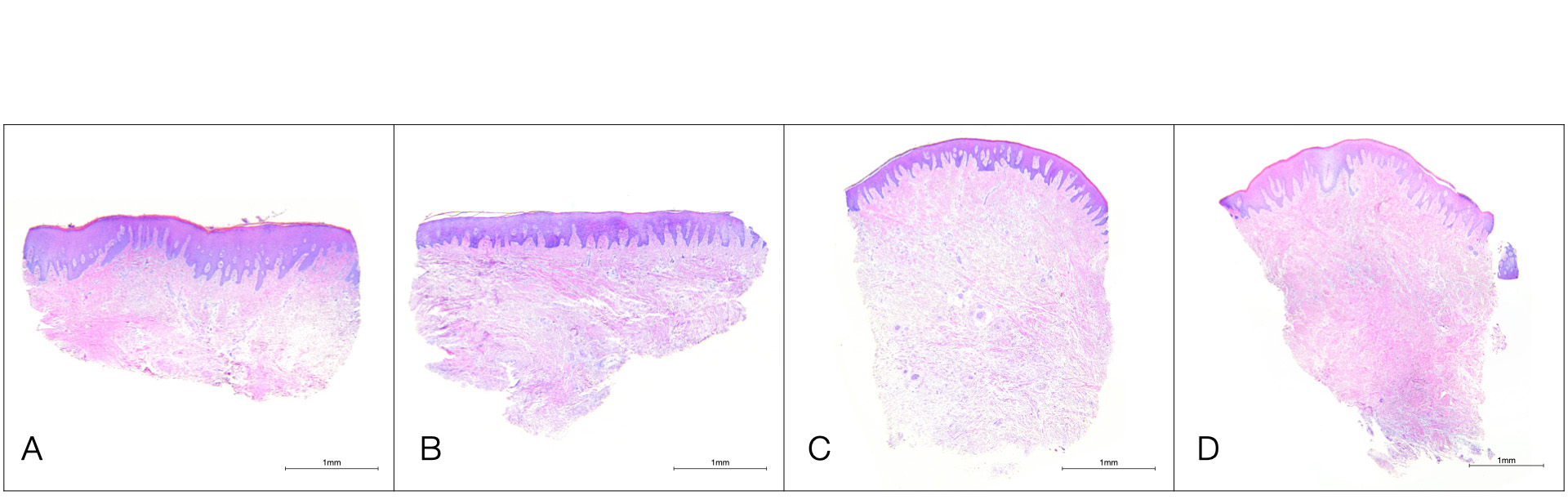
Figure 1. Photomicrographs of human tissue samples from different donor sites (hematoxylin and eosin staining). A) Anterior Palate; B) Posterior Palate; C) Maxillary Tuberosity, D) Retromolar Pad.Additionally, it has been demonstrated that there are biologic differences in terms of gene expression between various intraoral donor sites, with maxillary tuberosity and retromolar pad sites exhibiting higher expression of genes related to collagen biosynthesis and cell signaling compared to the lateral palate (Stuhr et al., 2023). Enzymes, such as lysyl hydroxylase (LH), and lysyl oxidase (LO), and their collaborative glycoproteins, such as thrombospondin 2 (THBS2), have been hypothesized to play a significant role in optimizing and regulating collagen cross-linking and maturation (Figure 2).
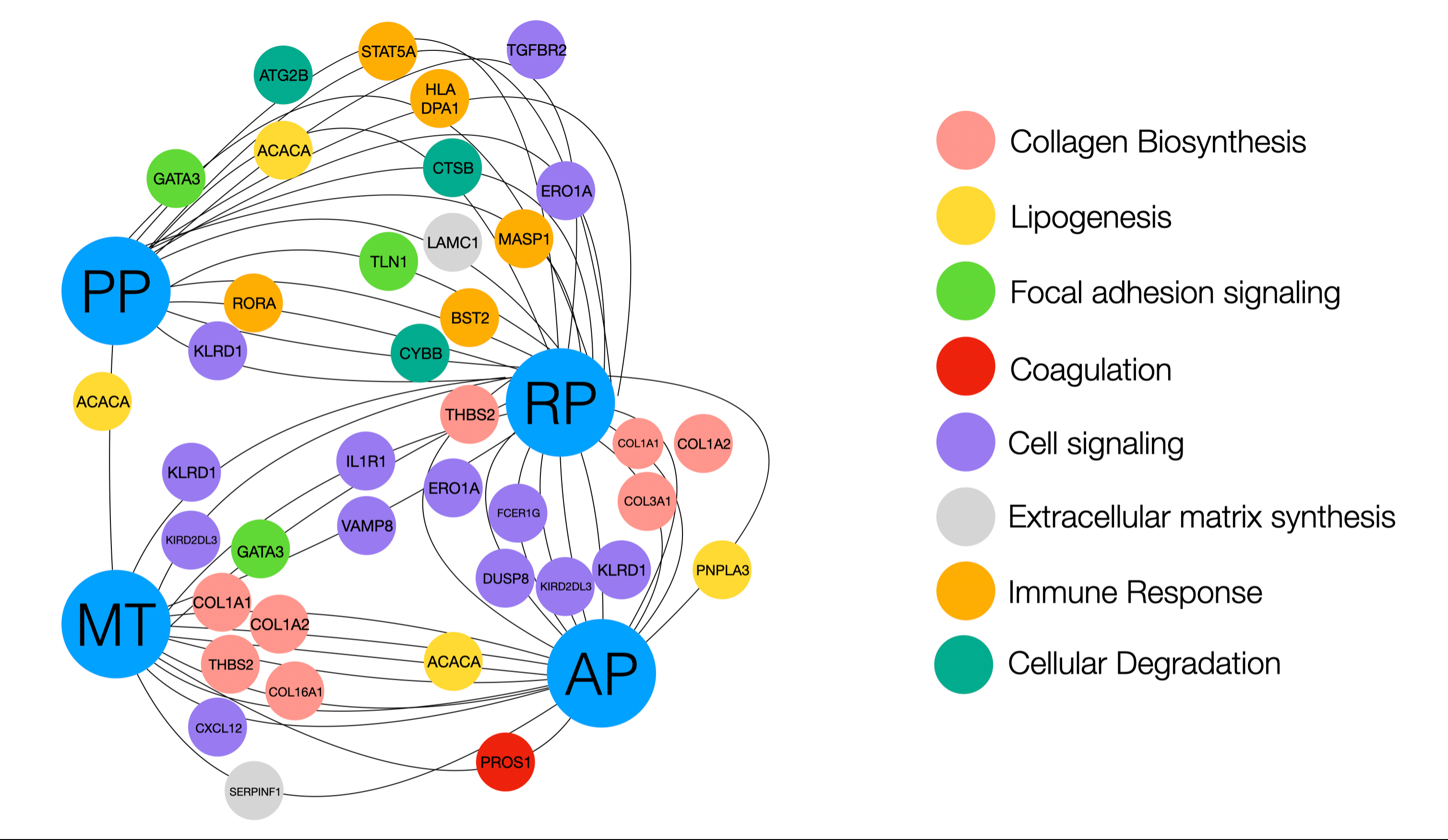
Figure 2. Illustration displaying genes that were shown to be significantly overexpressed or underexpressed. Genes labeled closer to the donor site (labeled in blue) were more highly expressed, and genes that are farther away were underexpressed compared to the opposing donor site. AP, anterior palate; PP, posterior palate; MT, maxillary tuberosity; RP: retromolar pad (Reprinted with permission from Stuhr et al. 2023).
| Donor site | Biological Considerations | Clinical Advantages | Clinical Disadvantages |
| Anterior/Posterior Palate | More abundant submucosal layer compared to tuberosity or retromolar pad
Angiogenesis and lipogenesis are more marked than collagen biosynthesis and cell signaling |
Ideal morphology and length for soft tissue grafting around teeth presenting non-proximal gingival recession defects.
Favorable access for harvesting
Ample variety of harvesting techniques |
Limited tissue availability in shallow palatal vaults
Greater risk of intraoperative and post-operative complications (e.g., bleeding, donor site morbidity) |
| Tuberosity/Retromolar Area | Thicker lamina propria compared to anterior/posterior palate
Collagen biosynthesis and cell signaling are more marked than angiogenesis and lipogenesis |
Ideal morphology for single unit peri-implant soft tissue augmentation or pontic site development
Lower risk of intraoperative and post-operative complications (e.g., bleeding, donor site morbidity) |
Quantity could be inadequate depending on therapeutic needs and anatomical features (i.e., presence of third molars)
Difficult access and technical execution, particularly in tuberosity regions
Limited applicability in certain scenarios (i.e., multiple gingival recession defects).
|
Table 1. Biological considerations and clinical advantages / disadvantages of harvesting CTG according to the donor site
In summary, clinicians should understand that the phenotypic heterogeneity and proliferative properties of the oral soft tissues may play a role in the clinical outcomes of soft tissue augmentation procedures (Table 1). The magnitude of such differences is also shaped by other factors such as the patient’s overall health status, clinician experience level, surgical techniques utilized, and the wound healing process. Each patient presents a unique biological environment, necessitating a personalized strategy to optimize long-term success. The following section will delve specifically into the technical nuances of various harvesting techniques, examining the methodologies and ways they align with the foundational biological principles already addressed.
HARVESTING TECHNIQUES
When deciding on the donor site for harvesting autogenous connective tissue grafts, clinicians should consider not only the biological properties of the donor site but also the specific clinical context. The decision should be based on factors such as recipient site characteristics, donor site morphology, quantity of available tissue, the technique employed, the anticipated healing outcomes after soft tissue augmentation, and, of course, the therapeutic goal(s). In daily clinical practice, the most common indications of CTGs are root coverage, correction of peri-implant soft tissue dehiscences and soft tissue volume gain in esthetic regions.
As a forementioned, CTGs may be obtained from the anterior palate, posterior palate, maxillary tuberosity and retromolar pad. Although the most sourced area has traditionally been the palatal mucosa between the distal line angle of the maxillary canine to the distal aspect of the first molar, followed by the tuberosity region, each donor region is associated with a series of clinical advantages and disadvantages, as summarized in Table 1.
Different technical approaches for CTG harvesting have been described.
Direct approach
This approach aims at obtaining a CTG with no epithelial remnants by making a series of intraoral incisions.
The classic approach, also known as the trap door access, consists of a single parallel longitudinal mesiodistal incision followed by two vertical incisions at each end. Subsequently, a partial- or full-thickness flap can be elevated to harvest the underlying lamina propria with or without submucosa through sharp dissection. As shown in Figure 3, other technical modifications can also be applied, such as :
- An L-shaped access avoiding one of the vertical incisions
- A dual parallel incision method with a double-blade scalpel
- A single linear incision followed by internal subepithelial dissection
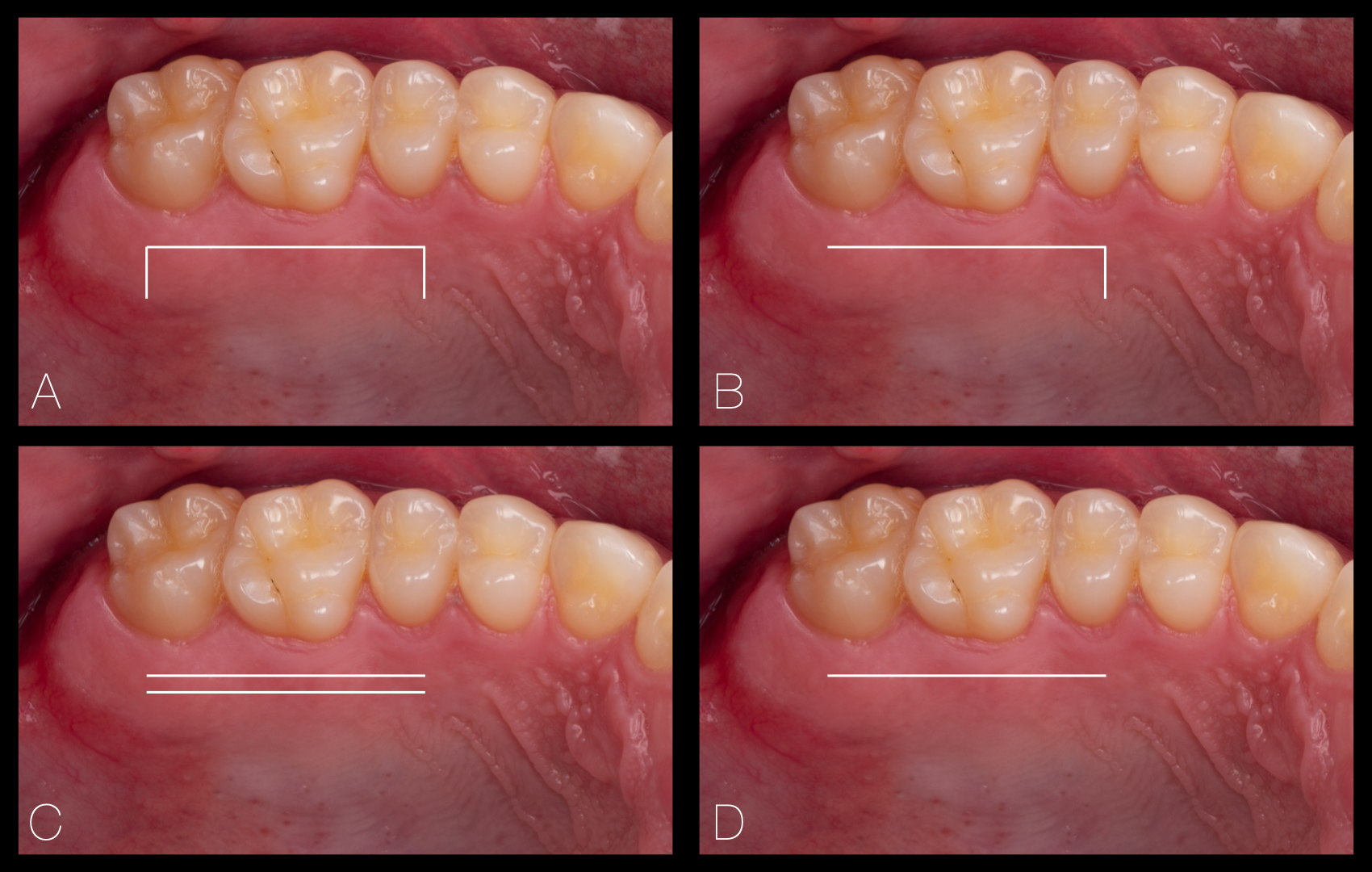
Figure 3. Mosaic depicting different methods to harvest a CTG: A) conventional trap door access; B) L-shaped access; C) Dual parallel-incision; D) Single-incision technique.
Like in the process of harvesting a free gingival graft, the most coronal longitudinal incision to obtain a CTG should be made at least 1mm apical from the base of the palatal gingival sulcus. Primary wound closure can be achieved in most instances after a direct harvesting approach.
Indirect approach
This approach consists of the de-epithelialization of the mucosal graft either intraorally with the use of a bur prior to extracting the graft or extraorally with a scalpel once the mucosal graft has been harvested, as depicted in Figure 4
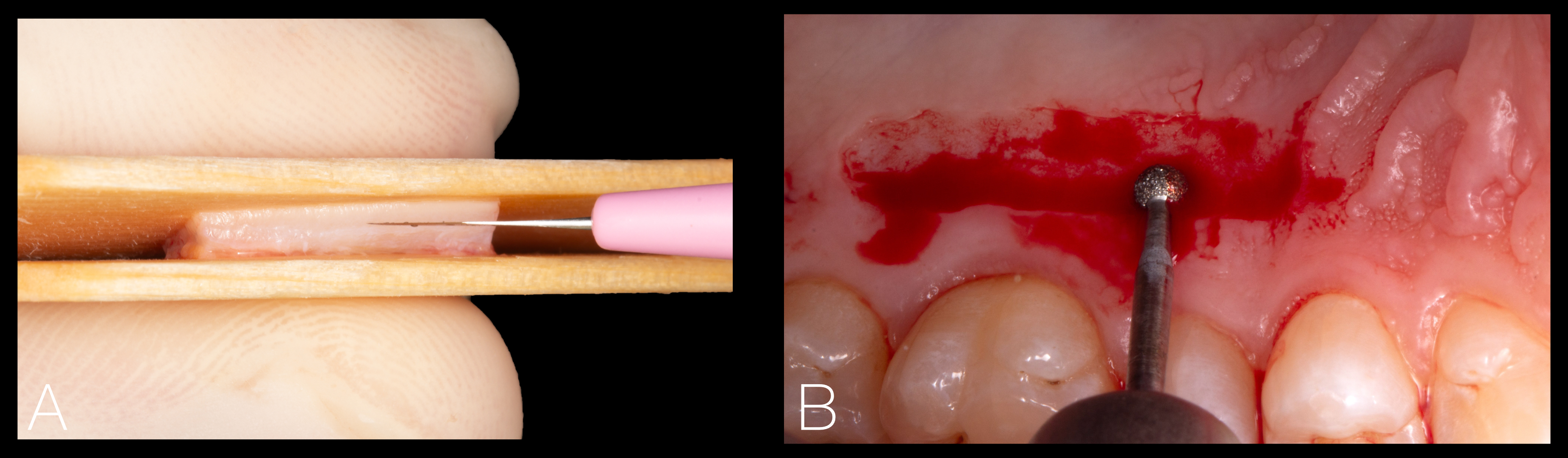
Figure 4. Extraoral de-epithelialization of a free mucosal graft using a fresh surgical blade (A), and intraoral de-epithelialization of a mucosal graft using a round diamond bur mounted on a high-speed handpiece (B).
A recent study has shown that the intraoral de-epithelialization method is slightly more effective in removing the epithelial layer and offers a series of advantages, such as time efficiency, reduced cost, enhanced visualization, greater preservation of the lamina propria and easier epithelium removal in non-flat areas (Couso-Queiruga et al., 2023). Grafts from the tuberosity and retromolar region may be harvested following the principles of the distal wedge technique or with a gingivectomy approach, as shown in Figure 5.
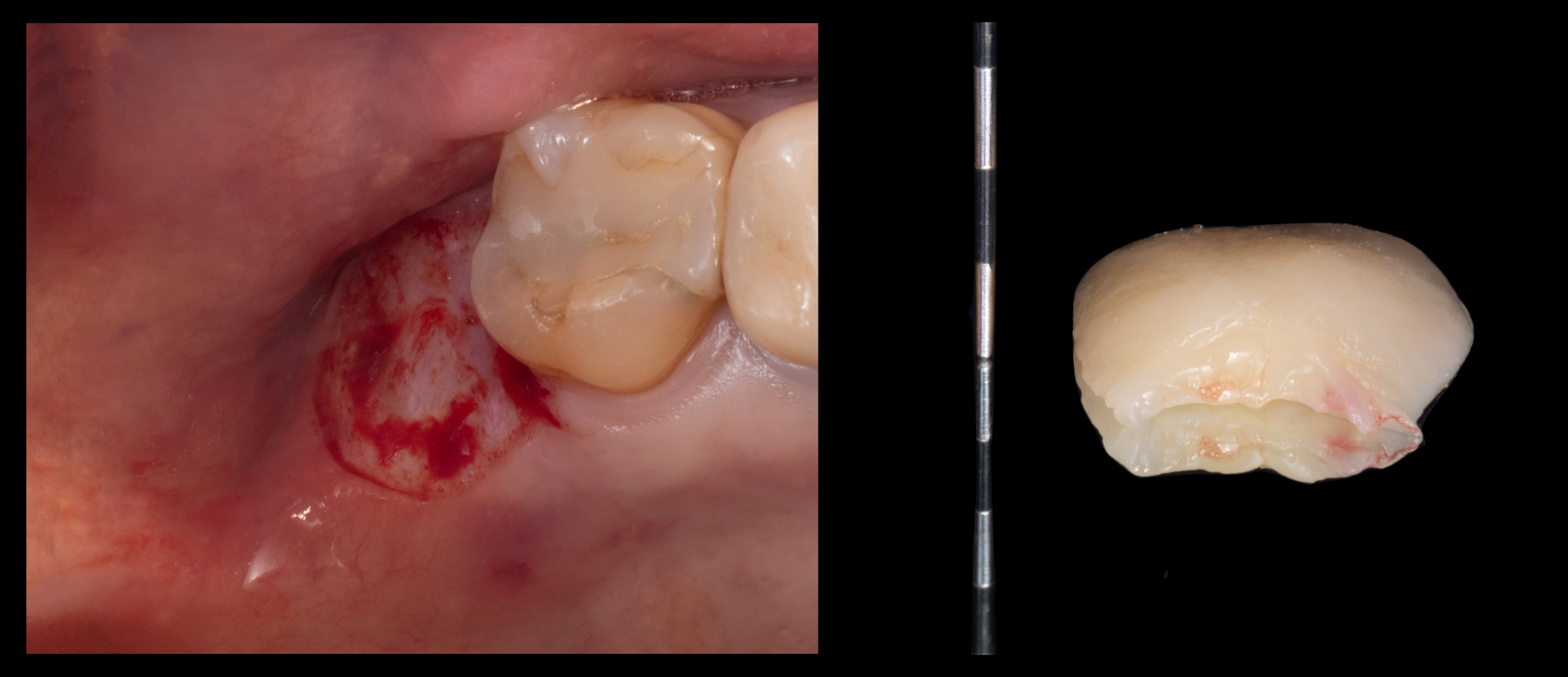
Figure 5. Free mucosal graft harvested from the tuberosity region with a gingivectomy utilizing a 12D scalpel. In the context of bilaminar techniques, it is crucial to remove the epithelium in the areas to be submerged under the flap.
Partially epithelialized soft tissue grafts can be also used in certain clinical situations (e.g., root coverage or extraction socket seal), but leaving the epithelialized portion intentionally exposed to the oral cavity. However, in cases where the CTG is to be completely submerged, any epithelial remnants should be removed to avoid complications, such as cyst formation.
Various methods can be utilized to protect the donor area after harvesting a CTG. These strategies may include the application of flowable composite, surgical glue (e.g., cyanoacrylate), the placement of dressings made of collagen or oxidized cellulose, sutures and/or the adaptation of a customized palatal stent to cover the wound. An example of two different methods to protect the donor site is depicted in Figure 6.
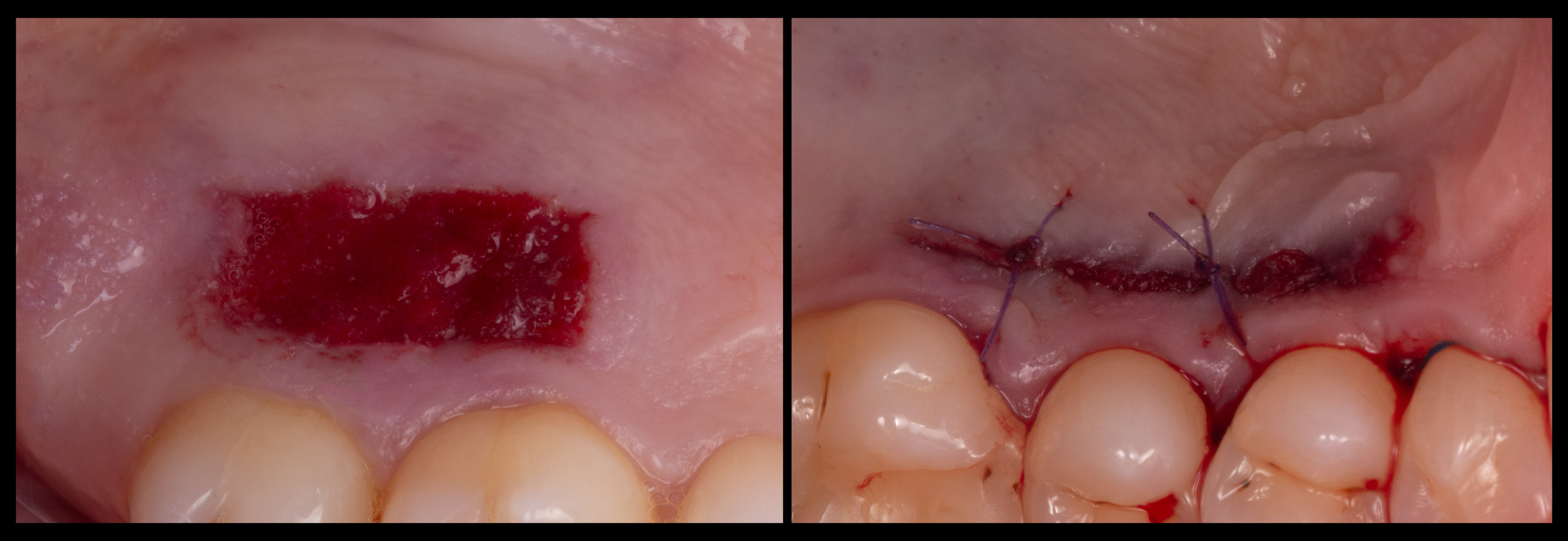
Figure 6. Donor sites treated with a collagen sponge secured with cyanoacrylate (left), or with a combination of cyanoacrylate and single absorbable sutures.
CONCLUSION
Autogenous soft tissue grafts represent a valuable resource in the context of mucogingival surgery to correct and prevent periodontal and peri-implant deformities. CTGs have specific indications to treat a wide variety of clinical entities, including the correction of single and multiple gingival recession defects. A deep understanding of the structural and biological properties of different intraoral donor sites, as well as recognizing the clinical advantages and disadvantages of distinct harvesting techniques are essential to optimize the execution of the surgical technique and increase the chances of achieving a satisfactory outcome.
BIBLIOGRAPHIE
- Chambrone, L., & Avila-Ortiz, G. (2022). Chapter 4: The Grafts. In L. Chambrone & G. Avila-Ortiz (Eds.), TISSUES: Critical Issues in Periodontal and Implant-Related Plastic and Reconstructive Surgery (pp. 206-314). Chicago: Quintessence Publishing.
- Chambrone, L., Botelho, J., Machado, V., Mascarenhas, P., Mendes, J. J., & Avila-Ortiz, G. (2022). Does the subepithelial connective tissue graft in conjunction with a coronally advanced flap remain as the gold standard therapy for the treatment of single gingival recession defects? A systematic review and network meta-analysis. J Periodontol, 93(9), 1336-1352. doi:10.1002/JPER.22-0167
- Chambrone, L., Ortega, M. A. S., Sukekava, F., Rotundo, R., Kalemaj, Z., Buti, J., & Prato, G. P. P. (2019). Root coverage procedures for treating single and multiple recession-type defects: An updated Cochrane systematic review. J Periodontol, 90(12), 1399-1422. doi:10.1002/JPER.19-0079
- Chambrone, L., & Tatakis, D. N. (2015). Periodontal soft tissue root coverage procedures: a systematic review from the AAP Regeneration Workshop. J Periodontol, 86(2 Suppl), S8-51. doi:10.1902/jop.2015.130674
- Couso-Queiruga, E., Gonzalez-Martin, O., Stuhr, S., Gamborena, I., Chambrone, L., & Avila-Ortiz, G. (2023). Comparative histological evaluation of intra- and extraorally de-epithelialized connective tissue graft samples harvested from the posterior palate region. J Periodontol, 94(5), 652-660. doi:10.1002/JPER.22-0493
- Dellavia, C., Ricci, G., Pettinari, L., Allievi, C., Grizzi, F., & Gagliano, N. (2014). Human palatal and tuberosity mucosa as donor sites for ridge augmentation. Int J Periodontics Restorative Dent, 34(2), 179-186. doi:10.11607/prd.1929
- Gamborena, I., & Avila-Ortiz, G. (2021). Peri-implant marginal mucosa defects: Classification and clinical management. J Periodontol, 92(7), 947-957. doi:10.1002/JPER.20-0519
- Garcia-Caballero, L., Gandara, M., Cepeda-Emiliani, A., Gallego, R., Gude, F., Suarez-Quintanilla, J., . . . Blanco-Carrion, J. (2023). Histological and histomorphometric study of human palatal mucosa: Implications for connective tissue graft harvesting. J Clin Periodontol, 50(6), 784-795. doi:10.1111/jcpe.13800
- Pohl, S., & Gluckman, H. (2024). Management of hyperplastic tissue response following connective tissue grafting. J Esthet Restor Dent. doi:10.1111/jerd.13232
- Sanz-Martin, I., Rojo, E., Maldonado, E., Stroppa, G., Nart, J., & Sanz, M. (2019). Structural and histological differences between connective tissue grafts harvested from the lateral palatal mucosa or from the tuberosity area. Clin Oral Investig, 23(2), 957-964. doi:10.1007/s00784-018-2516-9
- Stuhr, S., Nor, F., Gayar, K., Couso-Queiruga, E., Chambrone, L., Gamborena, I., . . . Ganesan, S. M. (2023). Histological assessment and gene expression analysis of intra-oral soft tissue graft donor sites. J Clin Periodontol, 50(10), 1360-1370. doi:10.1111/jcpe.13843
- Tavelli, L., Barootchi, S., Avila-Ortiz, G., Urban, I. A., Giannobile, W. V., & Wang, H. L. (2020). Peri-implant soft tissue phenotype modification and its impact on peri-implant health: A systematic review and network meta-analysis. J Periodontol. doi:10.1002/JPER.19-0716
- Zucchelli, G., Tavelli, L., McGuire, M. K., Rasperini, G., Feinberg, S. E., Wang, H. L., & Giannobile, W. V. (2020). Autogenous soft tissue grafting for periodontal and peri-implant plastic surgical reconstruction. J Periodontol, 91(1), 9-16. doi:10.1002/JPER.19-0350